Food and Drug Administration issued an emergency use authorization EUA for the second vaccine for the prevention of. By edhat staff Today the US.
The FDA amended the emergency use authorization originally issued on Dec.

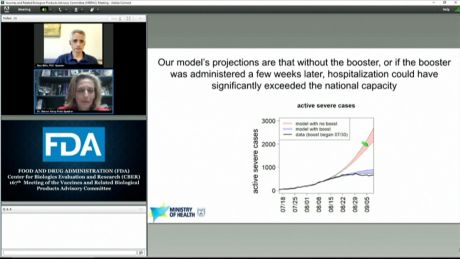
Fda booster decision. Although vaccine companies are already in the process of conducting clinical trials for booster shots and preparing for potential widespread distribution a decision pertaining to. On December 18 2020 the US. Choosing to participate in a study is an important personal decision.
If approved Biogens aducanumab would be the first treatment to address an underlying cause of the memory-robbing condition which is the sixth. The South African Health Products Regulatory Authority issued a statement saying it had reviewed the data provided by the FDA and has made a decision not. If regulators agree with Pfizer s conclusions and briefing documents suggest that s a big if about real-world studies showing Comirnaty s effectiveness waning over time thereby justifying the need for a booster dose it could be part of a historical moment for FDAs use of RWE.
Immunity Duration of Rabies Vaccine and Booster Dose Effects at 10 Years Post-primary Vaccination. The US Food and Drug Administration FDA is adding a warning to the fact sheets for the PfizerBioNTech and Moderna mRNA COVID-19 vaccines as. The FDAs decision may be viewed as a barometer for how strict the agency will be under the Biden administration which has yet to name an FDA commissioner.
The COVID-19 death toll in the US. Regulators are slated to decided by Monday whether to approve Biogen Incs controversial Alzheimers disease drug and Wall Street analysts and industry observers are deeply divided on its chances of making it over the finish line. With that advice the FDA is expected to make a final decision within days.
Talk with your doctor and family members or friends about deciding to join a study. The FDA is facing a no-win decision on Biogens Alzheimers treatment aducanumab. JCVI COVID-19 Chair Professor Wei Shen Lim said in a statement.
Biogen has responded to questions from the Alzheimers disease community regarding its Eisai-partnered anti-amyloid drug Aduhelm as recently revealed memos show a struggle within the US Food and Drug Administration FDA over the drugs approval. After collaborating with the company on review of its approval application in spite of mixed and controversial data a panel of outside advisers voted decisively against the drugs benefit-risk profile. Information about the Moderna COVID-19 Vaccine.
Nearly five years later the drugs benefits have still not been confirmed by an FDA-mandated follow-up. 11 2020 for administration in individuals 16 years of age and older. Food and Drug Administration FDA expanded the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 to include adolescents 12 through 15 years of age.
Her official decision concluded the drug was reasonably likely to benefit some patients. The FDAs decision may be viewed as a barometer for how strict the agency will be under the Biden administration which has yet to name an FDA commissioner. FDA lifts clinical hold on bluebird bios LentiGlobin studies Company previously put trials on hold after suspected unexpected serious adverse reactions The US Food and Drug Administration FDA has lifted the clinical holds on numerous studies evaluating its gene therapy LentiGlobin for sickle cell disease SCD and beta-thalassemia.
Aduhelm is the first federally approved Alzheimers treatment in roughly 18 years but there is no conclusive evidence the drug slows the decline of memory and brain functionGet market news worthy of your time with Axios. The primary objective of any potential COVID-19 booster vaccine programme should be. Pfizer bags its first approval in the US for Prevnar 20 its new 20-valent pneumococcal conjugate vaccine a few weeks ahead of an FDA decision on Mercks rival V114.
Biogen is charging 56000 per year for the drugWhy it matters. The FDA has approved Biogens Alzheimers drug aducanumab which will be marketed as Aduhelm. The FDA has defended its decision by saying that Biogen presented clear evidence that Aduhelm known chemically as aducanumab removed beta.
Topped 500000 this week and the vaccination drive has been slower than hoped. Vaccines and Related Biological Products Advisory Committee Meeting December 10 2020 FDA Briefing Document Pfizer-BioNTech COVID-19 Vaccine Sponsor.
Fda S Decision On Pfizer Covid Booster Shots Shows Danger Of Publicizing Deadlines In A Crisis
How Fauci Got Ahead Of Fda And Cdc In Backing Covid Booster Shots Los Angeles Times